Printable Version of Topic
Click here to view this topic in its original format
914World.com _ 914World Garage _ diaphram analysis is in and ......
Posted by: Bleyseng Feb 27 2006, 02:19 PM
You were correct....COPPER.
--------
Ok, did the marterial analysis on the OEM diaphram this morning using a Niton XL-II 800 handheld alloy analyzer.
I made three different scans of the sample piece I got from Geoff. The readings were as follows:
#1 - 99.78% Cu
#2 - 99.20% Cu
#3 - 99.39% Cu
No berillium. Supposedly anything between 98%-100% copper is considered pure.
The remainder of the 100% reading was a spattering of Al, Si Br, Phos Brz.
--------------------
Eric
'75 914 2.0L
Posted by: Jeff Bowlsby Feb 27 2006, 02:36 PM
Pure copper eh? I am surpised that pure copper would be strong enough to withstand the constant fluxuations. Thats good news Eric (and Geoff), copper sheet is readily available.
![]() Now where is that press....
Now where is that press....
Posted by: Mueller Feb 27 2006, 02:51 PM
I still say it's BeCu......
Add your % findings to the % composition for second type.....
| QUOTE |
| Beryllium Copper -------------------------------------------------------------------------------- Overview Copper beryllium alloys are used for their high strength and good electrical and thermal conductivities. There are two groups of copper beryllium alloys, high strength alloys and high conductivity alloys. The wrought high strength alloys contain 1.6 to 2.0% beryllium and approximately 0.3% cobalt. The cast, high-strength alloys have beryllium concentrations up to 2.7%. The high conductivity alloys contain 0.2-0.7% beryllium and higher amounts of nickel and cobalt. These alloys are used in applications such as electronic connector contacts, electrical equipment such as switch and relay blades, control bearings, housings for magnetic sensing devices, non sparking applications, small springs, high speed plastic molds and resistance welding systems. Cast beryllium coppers are frequently used for plastic injection molds. The cast materials have high fluidity and can reproduce fine details in master patterns. Their high conductivity enables high production speed, while their good corrosion and oxidation resistance promotes long die life. The UNS designations for the wrought alloys are C17200 through C17400 and the cast alloys are C82000 through C82800. The high strength of the copper beryllium alloys is attained by age hardening or precipitation hardening. The age or precipitation hardening results from the precipitation of a beryllium containing phase from a supersaturated solid solution of mostly pure copper. The precipitation occurs during the slow cooling of the alloys because the solubility of beryllium in alpha copper decreases with decreasing temperature. Typically the alloys are rapidly cooled from the annealing treatment, so the beryllium remains in solid solution with the copper. Then the alloy is given a precipitation or age hardening treatment for an hour or more at a temperature between 200 and 460 C. Upon tempering, the beryllium containing phases, called beryllides, precipitate out of solution. |
More reading here:
http://www.copper.org/resources/properties/microstructure/be_cu.html
Posted by: ClayPerrine Feb 27 2006, 07:16 PM
"Diaphram Analysis"....
That title leaves things wide open for some really low comments.....
I am suprised (and a bit disappointed) that no one chose to jump on that!
![]()
Posted by: lapuwali Feb 27 2006, 07:26 PM
What Mueller said. Alloying elements are sometimes found in astonishingly small percentages, yet can make a huge difference to how the material behaves. Now, it's quite possible one could get a useful diaphram made from nearly pure copper, though I'd be willing to bet it wouldn't work nearly as well over wide temperature ranges, which I believe is what the Be was originally put there for: small thermal expansion coefficient.
Posted by: alpha434 Feb 27 2006, 07:30 PM
A BeCu alloy usually ranges from 0.25% to 2.00% Be.
This falls within the range that was measured, yeah?
Posted by: RustyWa Feb 27 2006, 07:30 PM
| QUOTE (Mueller @ Feb 27 2006, 04:35 PM) |
| unless I mistaken, that analyzer will not pickup Be according to the spec sheet...........(unless tested in a vacuum) |
That could turn out to be a big bummer. I'll explore the analyzer literature a little more and talk to some other engineers about it.
Posted by: tdgray Feb 27 2006, 07:32 PM
I second that respond.
I run a forging operation. If there is one thing I see constantly is the effect of "small" element variations on our work.
Take 1045 steel... normal specs call for anthing from 1042 to 1048 to fall within the range of 1045. But, use the variant on some jobs and you will find siginificant problems with flash extension and grain flow.
I do not profess to know much about steel chemistry but I do know from mixed steel that certain elements can mimic other compositions.
Could this be the case here. ![]()
Posted by: Mueller Feb 27 2006, 07:32 PM
| QUOTE (alpha434 @ Feb 27 2006, 06:30 PM) |
| A BeCu alloy usually ranges from 0.25% to 2.00% Be. This falls within the range that was measured, yeah? |
thanks for repeating what I've already posted a few hours ago
Posted by: kwales Feb 27 2006, 07:34 PM
Did you try Rockwell hardness?
Might ID the material that way..
Ken
Posted by: alpha434 Feb 27 2006, 07:35 PM
And .02% cobalt-nickel used as a stabilizer.
You didn't get and cobalt or nickle, did ya?
My bet is that it isn't BeCu.
Posted by: tdgray Feb 27 2006, 07:39 PM
Oh great another bet from the aplha dog... ![]()
Rockwell IIRC cannot determine chemical composition. Just suggest at it. Same as spark testing etc.
Posted by: alpha434 Feb 27 2006, 07:41 PM
| QUOTE (tdgray @ Feb 27 2006, 05:39 PM) |
| Oh great another bet from the aplha dog... Rockwell IIRC cannot determine chemical composition. Just suggest at it. Same as spark testing etc. |
Yeah.
Wanna bet?
I KNOW I'm right here. This is my area. I had to write a four page report on Cupric alloys for school.
Posted by: tdgray Feb 27 2006, 07:45 PM
Yes I would wanna bet little shaver.
If I give you a raw piece of steel...then you can give me the chemical composition of the steel...
Jesus you must be F'in magic. I will hire you as the Chief metalurgist at my plan if you can do that.
![]()
Posted by: alpha434 Feb 27 2006, 07:46 PM
it's 1080.
And you couldn't afford me.
Posted by: alpha434 Feb 27 2006, 07:47 PM
The initial readings were given at the top of this thread.
If there is no nickel and no cobalt, then it will not be BeCu.
Posted by: kwales Feb 27 2006, 07:51 PM
Ok boys,
I cut and pasted the tester into a search engine and found..... (drumroll please..)
The tester used doesn't have Beryllium as a listed material that it can detect- with ANY isotope.
Rockwell is a dumb easy practical test that can be used to narrow the materials. Depending on the results, you can eliminate a number of materials and hardnesses. (this one is too soft, this one is too hard, this one is JUST right)
Alpha, what are ya gonna do if you have two different Cu materials/treatments that have the same hardness... You won't be able to get alloys by hardness. You can narrow the possibilities but other tests are needed.
Ken
Posted by: alpha434 Feb 27 2006, 07:55 PM
You can usually tell JUST by machinability. BeCu chips are kinda powdery, and its easy to get a good finish. Cu comes off like chewying gum.
You don't have a large enough piiece to machine.
You can't chemically test it for fear of realeasing the Be.
I dunno! That one's tough. I'll brainstorm.
Posted by: Aaron Cox Feb 27 2006, 07:57 PM
i rockwell tested my hootus... it said it was hard ![]()
Posted by: Mueller Feb 27 2006, 07:57 PM
| QUOTE (kwales @ Feb 27 2006, 06:51 PM) |
| Ok boys, I cut and pasted the tester into a search engine and found..... (drumroll please..) The tester used doesn't have Beryllium as a listed material that it can detect- with ANY isotope. Rockwell is a dumb easy practical test that can be used to narrow the materials. Depending on the results, you can eliminate a number of materials and hardnesses. (this one is too soft, this one is too hard, this one is JUST right) Alpha, what are ya gonna do if you have two different Cu materials/treatments that have the same hardness... You won't be able to get alloys by hardness. You can narrow the possibilities but other tests are needed. Ken |
I posted that on the other thread on this subject...the tester "might" be able to find Bu if used in a vac.
how much movement does a diaphram see?
wouldn't be too hard to just make a fixture to run some sample cycle tests of differing alloys
Posted by: bd1308 Feb 27 2006, 07:59 PM
ah...hell i'll test it
I've literally probably eaten any one person's fair share of lead from using solder and stuff....i'll light it on fire for ya'll....
Na+H2O=BOOM
b
Posted by: alpha434 Feb 27 2006, 08:02 PM
It's a stamped piece, right?
Be is ONLY added for machinability. If this wasn't a machined piece, then its, from a manufacturing perspective, 99% pure. Like you said. Same hardness. Most other mechanical features should be the same. Cu has a tendency to cold flow under extreme pressure. BeCu will crack.
Posted by: tdgray Feb 27 2006, 08:03 PM
| QUOTE (alpha434 @ Feb 27 2006, 09:46 PM) |
| it's 1080. And you couldn't afford me. |
What's 1080...
And believe me I am sure we could afford you... just not the rubber room we'd have to house you in.
Aaron what what the reading on your hootus... inquiring minds wanna know...
And for all those following the story... you cannot tell chemical (elemental) analysis of steel by rockwell testing. My V.P. of QC (who owns a 914 and visits this board) can tell you that.
Posted by: tdgray Feb 27 2006, 08:05 PM
| QUOTE (alpha434 @ Feb 27 2006, 10:02 PM) |
| Be is ONLY added for machinability. If this wasn't a machined piece, then its, from a manufacturing perspective, 99% pure. Like you said. |
OMHFMOG....
Anyone want to field this one...
Bueller... Bueller...
That's it for me tonight folks. Feel free to keep it up
Posted by: alpha434 Feb 27 2006, 08:05 PM
| QUOTE (bd1308 @ Feb 27 2006, 05:59 PM) |
| ah...hell i'll test it I've literally probably eaten any one person's fair share of lead from using solder and stuff....i'll light it on fire for ya'll.... Na+H2O=BOOM b |
The acceptable environmental tolerence for Be is a pinch spread across a football field evenly.
A shop I worked for had to use a piece for fitting another part too. Some guy dropped it, and everyone literally sprinted to the door.
Posted by: MattR Feb 27 2006, 08:06 PM
| QUOTE (tdgray @ Feb 27 2006, 06:03 PM) |
| Aaron what what the reading on your hootus... inquiring minds wanna know... |
98% stud
Posted by: Aaron Cox Feb 27 2006, 08:06 PM
| QUOTE (tdgray @ Feb 27 2006, 07:03 PM) |
| Aaron what what the reading on your hootus... inquiring minds wanna know... |

| QUOTE (MattR @ Feb 27 2006, 07:06 PM) | ||
98% stud |
yep
Posted by: tdgray Feb 27 2006, 08:09 PM
NIIIIIIICCCCCEEEEE.
![]() I couldn't find the DAAAAAMMMMNNN banner.
I couldn't find the DAAAAAMMMMNNN banner.
Thanks for portraying the lousy posting by me guys... thats real special ![]()
Posted by: alpha434 Feb 27 2006, 08:10 PM
| QUOTE (tdgray @ Feb 27 2006, 06:05 PM) | ||
OMHFMOG.... Anyone want to field this one... Bueller... Bueller... That's it for me tonight folks. Feel free to keep it up |
So. Am I wrong because I'm wrong? Or am I wrong because I'm young. If you KNOW better than this, feel free to post whatever data you have. Because up until now, you haven't added much. A lotta blank space.
If I think of a way to test it safely, I'll pm one of you guys. I'm off this thread.
Posted by: bd1308 Feb 27 2006, 08:13 PM
The EPA restricts the amount of beryllium that industries may release into the air to 0.01 µg/m³, averaged over a 30-day period.
The Occupational Safety and Health Administration (OSHA) sets a limit of 2 µg/m³ of workroom air for an 8-hour work shift.
http://www.atsdr.cdc.gov/tfacts4.html
b
Posted by: balljoint Feb 27 2006, 08:28 PM
"Early researchers tasted beryllium and its various compounds for sweetness in order to verify its presence. Modern diagnostic equipment no longer necessitates this highly risky procedure and no attempt should be made to ingest this substance. "
So no volunteers to taste test original equipment?
Posted by: Mueller Feb 27 2006, 08:30 PM
| QUOTE (alpha434 @ Feb 27 2006, 07:05 PM) |
| A shop I worked for had to use a piece for fitting another part too. Some guy dropped it, and everyone literally sprinted to the door. |
sounds like a bunch of sissies
I have props for my RC boats made of the stuff, I can make a necklace out of it if I wanted to...just don't grind the stuff without proper ventalation and you are fine.........
Posted by: MattR Feb 27 2006, 08:35 PM
| QUOTE (Mueller @ Feb 27 2006, 06:30 PM) |
| I have props for my RC boats made of the stuff |
what class do you run?
Posted by: Mueller Feb 27 2006, 08:37 PM
| QUOTE (MattR @ Feb 27 2006, 07:35 PM) | ||
what class do you run? |
my boats are like my 914
no particluar class, just built a few different ones....gas and electric for fun....
Posted by: MattR Feb 27 2006, 08:41 PM
| QUOTE (Mueller @ Feb 27 2006, 06:37 PM) | ||||
my boats are like my 914 no particluar class, just built a few different ones....gas and electric for fun.... |
Cool.
But Ive never seen an RC boat on jackstands
Posted by: alpha434 Feb 27 2006, 08:43 PM
If you chip it from dropping it, it'll throw up loose particles. But my machine shop teacher said that he used to machine it all the time. Said they just did it under an oil bath. Now they have some REALLY strict limitations on it.
Posted by: lapuwali Feb 27 2006, 09:28 PM
| QUOTE (alpha434 @ Feb 27 2006, 06:02 PM) |
| It's a stamped piece, right? Be is ONLY added for machinability. If this wasn't a machined piece, then its, from a manufacturing perspective, 99% pure. Like you said. Same hardness. Most other mechanical features should be the same. Cu has a tendency to cold flow under extreme pressure. BeCu will crack. |
No, it's also added to improve conductivity, and change the thermal expansion coefficient. Be has a very low coefficient of expansion, where Cu has a fairly high coefficient. Since I'd expect an pressure measuring device would not want to be substantially affected by changes in temperature, I'd say the latter quality would be good to get under control.
Be MAY (I'm speculating here) also improve Cu's properties wrt work-hardening, since an MPS diaphram is also going to flex a great deal over many many cycles. The fact that they fail the way they do shows even BeCu isn't good enough, at least not for 30+ years of service...
Posted by: LvSteveH Feb 27 2006, 10:18 PM
| QUOTE (alpha434 @ Feb 27 2006, 06:10 PM) |
| So. Am I wrong because I'm wrong? Or am I wrong because I'm young. |
Sometimes just plain wrong is good enough..... this is just such a case. You make way too many broad generalizations and unsupported assertions that are clearly derived from having heard or read something that is only vaguely relevant. Here's a tip, any time you consider saying "always" or "none" or "never" you are setting yourself up for failure. These are lessons that do in fact come largely with age, so in a round-about way, maybe it is because you are young, although you are not being discredited on that basis. Your age is simply the mechanism for your folly. Age and wisdom by no means go hand in hand, but they do occasionally intersect.
Another good tip, don't ask questions you don't want to hear the answer to.
Posted by: Bleyseng Feb 27 2006, 11:03 PM
I want to hear how Aaron's hootus tests a diaphrams flexiblity...and what the maximum flex is especially with how short its travel is. ![]()
Damn, that copper diaphram tastes sweet!
Posted by: RustyWa Feb 28 2006, 02:06 PM
| QUOTE (alpha434 @ Feb 27 2006, 05:35 PM) |
| And .02% cobalt-nickel used as a stabilizer. You didn't get and cobalt or nickle, did ya? My bet is that it isn't BeCu. |
The analyzer I used, turns out, will not identify beryllium. That's my fault as I'm not a chemist nor a metallurgist. I just have access to this analyzer. It does do a real good job on nickel and cobalt and it did not list either of those in the scanning that I did yesterday and today.
Then again, maybe the half-life of the source is getting old and is pretty weak....
BeCu chemical composition: http://www.olinbrass.com/becu_chemical.html
Posted by: Mueller Feb 28 2006, 02:10 PM
| QUOTE (RustyWa @ Feb 28 2006, 01:06 PM) | ||
The analyzer I used, turns out, will not identify beryllium. That's my fault as I'm not a chemist nor a metallurgist. I just have access to this analyzer. It does do a real good job on nickel and cobalt and it did not list either of those in the scanning that I did yesterday and today. Then again, maybe the half-life of the source is getting old and is pretty weak.... |
you gave it your best shot so
Posted by: Demick Feb 28 2006, 03:24 PM
Eric
Do you know what Radioisotope source was used in the analyzer? Different sources detect different elements. If yours is set up mainly for detecting steels, then it won't be ideal for analyzing copper alloys, and doesn't look like it will detect tin at all. This is important because another possibility for the diaphragm material is phosphor bronze, which should have large amounts of tin.
Demick
Attached image(s)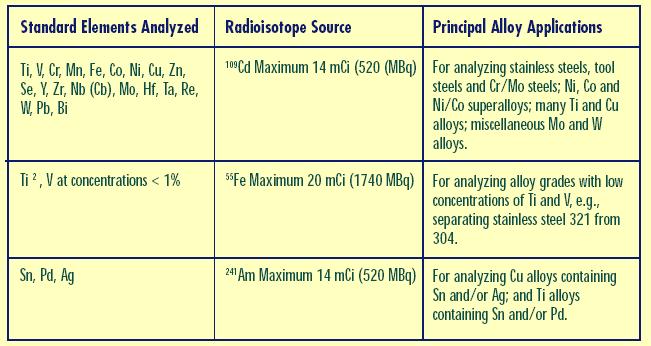
Posted by: RustyWa Feb 28 2006, 09:05 PM
I was just looking at the label's this afternoon, but I do not remember what it said. C-10 seems to stick in my mind, I'll look tomorrow and report back.
I do know it only has one radioisotope installed in the unit and we use it mainly for verifying piping, vessels, welds, etc. at the refinery to make sure the right material is being used.
If you look at my original post, you'll see the display also contained other information, for example:
99.78% Cu
Elec Cu
Al, Si Br
Phos Brz.
Posted by: Joe Ricard Feb 28 2006, 09:57 PM
Hootus hardness.... ![]() That's freaking funny I don't care who you are.
That's freaking funny I don't care who you are.
We shoould have a virtual Hootus hardness contest.
Like the 914Cup. everybody can make shit up about how hard there hootus is and how long it lasts.
Posted by: RustyWa Mar 4 2006, 09:54 PM
| QUOTE (Demick @ Feb 28 2006, 01:24 PM) |
| Do you know what Radioisotope source was used in the analyzer? |
CD-109 10mCi
Posted by: bd1308 Mar 5 2006, 10:26 AM
Well I called the Fuel Injection company (who bought out Bret Instruments) and the fellow over there, apparently the owner said that their diaphrams are copper, not a berillum-copper alloy. Or at least that what he conveyed by his explaination.
b
Posted by: Bleyseng Mar 5 2006, 10:59 AM
Well, that's better than the brass one sitting here on my desk.....
Posted by: kwales Mar 5 2006, 11:57 AM
I'm gonna ask one more time then I'm gonna shut up...
Has anyone tested the hardness?
Is it dead soft? Quarter hard? Half hard? Full hard?
Is it a spring or a maleable piece of material?
And.... if the hardness is known, you might REALLY narrow down yer choices of what materials it could be...
I also want to thank RustyWa for having the initiative to give it a try with his equipment at work. Sorry it didn't work out. My thanks to you again. It is effort like this that will get the material known ![]()
Back to the regularly scheduled program of "letstalkaboutitsomemore"
![]()
KenwithanLjet
Posted by: Bleyseng Mar 5 2006, 12:02 PM
Hopefully, Full Hard! ![]()
Posted by: bd1308 Mar 5 2006, 03:42 PM
can I help in anyway?
I have two good diapragms (OE) that I can use to help....
its a springy material, definately not something that would seem to work harden very easily (for obvious reasons)
b
Posted by: Bartlett 914 Mar 5 2006, 04:16 PM
I can probably measure the hardness in vickers. We use this in the gravure industry to measure the hardness of the copper plated cylinders we use. Maybe Britt can send me one of his. The test will not harm it. PM me if interested.
Posted by: bd1308 Mar 5 2006, 04:19 PM
vickers does make an indention, or is that the third type?
i'm game...lemme know where to send the diapragm.
b
Posted by: bd1308 Mar 5 2006, 04:21 PM
and from vickers we can get a approx Rockwell hardness....and from that we cna convert to Hootus Hardness.
it all works out.
we need a hootus standard though.... hmmm
b
Posted by: Bleyseng Mar 5 2006, 04:24 PM
Hootus standard:
Soft hard-no damn good
Full Hard- excellent!
![]()
Powered by Invision Power Board (http://www.invisionboard.com)
© Invision Power Services (http://www.invisionpower.com)